|
Neisser and Gram Staining!! |
|
Neisser staining Staining according to Neisser is a
test for the presence of polyphosphates stored in the cells (= storage
materials). This method is an indispensable aid to the identification of
certain strains of filamentous bacteria. Furthermore, this staining method can
make the Bio-P bacteria, responsible for biological phosphate removal, visible.
A. Methylene
blue
0.1 g B. Crystal
violet, 10% in 96% ethanol 3.3 ml C. Chrysoidin
Y, 1% aqueous solution 33.3 ml Distilled water
100 ml Staining procedure · Prepare
a fixed smear. · Place
a freshly made mixture of 2 parts solution A and 1 part solution B onto the
slide for a contact period of 10-15 seconds. Afterwards, allow the excess dye
to run off the slide. · Add
solution C for a contact period of 45 seconds. · Rinse
the slide with tap water (with the flow against the back of the slide). · Allow
the slide to dry and then view with a 100x bright field objective. Drying can
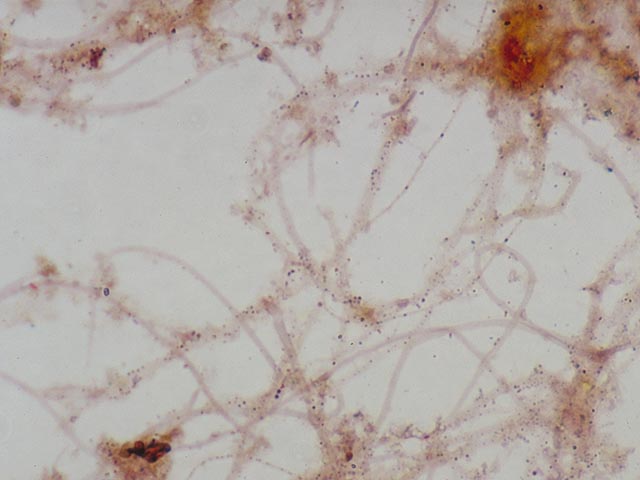
be speeded up by removing most of the water carefully with filter paper. Results Neisser negative cells stain hardly
or not at all (slightly brown or yellow; Three main groups of Neisser
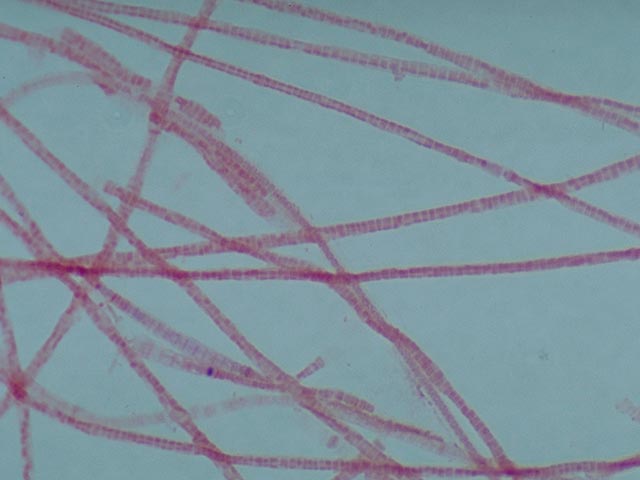
positive bacteria can be distinguished. 1. Filamentous bacteria which stain
completely grey-violet . This almost always applies to Nostocoida limicola or Type 0092. 2. Filamentous
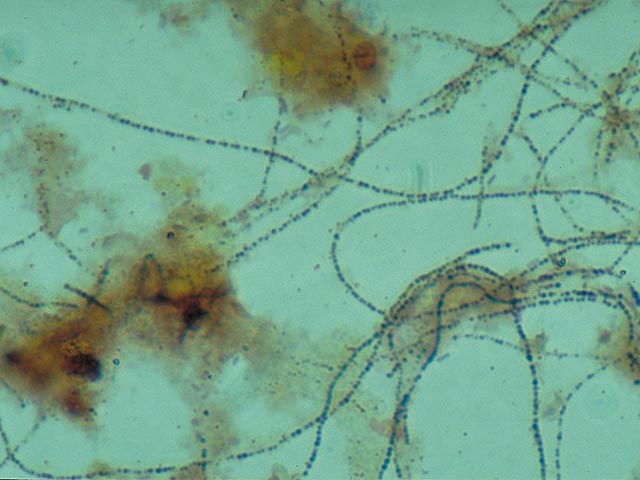
bacteria which contain
blue-black coloured polyphosphate globules . Without staining,
these
globules cannot be clearly observed with a light microscope. They are
indeed
clearly visible if a much higher magnification (electron microscopy) is
used . These globules, which are present in pairs, are an
important
identification characteristic for
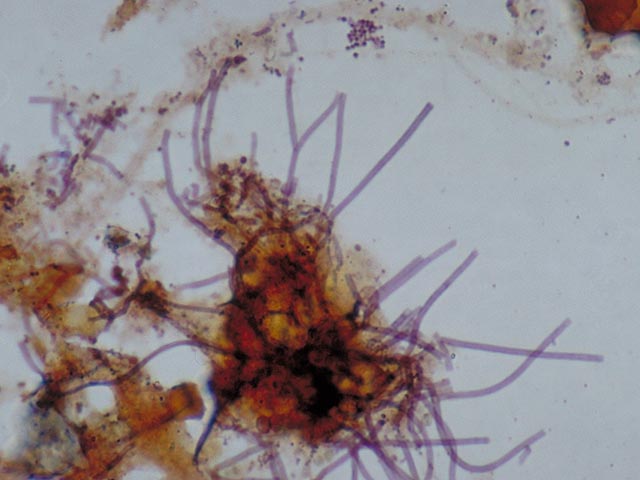
Microthrix parvicella. 3. Colonies of blue-black coloured cells . These are comprised of Bio-P bacteria. There are some variations in the manner in which these types of colonies stain with Neisser. The shade is sometimes much lighter , or only a part of the cell stains darkly.
Back to Top
Gram Staining Gram staining is an indispensable
aid when identifying bacteria. This staining first colours the bacteria blue
using carbol gentian violet. The cells are then washed with an alcohol
solution. The cells of some bacterial strains re-release the absorbed blue dye
during this process. These bacteria are known as Gram negative. In the case of
Gram positive bacteria, the absorbed carbol gentian violet cannot be removed by
washing with alcohol. The colourless Gram negative bacteria are subsequently
restained with safranine, which gives them a red colour. This is the result of
differences between Gram positive and Gram negative bacteria in the composition of the cell wall. Necessary solutions A Carbol gentian violet solution Dilute 10 ml of the stock
solution with 90 ml of a 5% phenol
solution. Stock
solution (Carbol
gentian violet 10 g, alcohol
(96%) 90 ml.) B. Lugol’s
iodine solution Dissolve
3 g KI in a few mls of distilled water, mix in 1 g I2 and dilute to 300 ml with distilled
water. C. Alcohol
solution
Dilute
7 ml of the stock solution with 1000 ml (96%) alcohol.
Stock solution ( I2 100
g , KI 40 g, alcohol (96%) 1250 ml distilled water 100 ml.) D. Safranine
solution Dissolve
0.25 g safranine in 10 ml (96%) alcohol and dilute with 100 ml distilled water. Staining procedure · Prepare
a fixed smear (see paragraph 2.3). · Apply
solution A for a contact period of 60 seconds; subsequently allow the excess
dye to run off the slide. · Apply
solution B for a contact period of 60 seconds; subsequently allow the excess
dye to run off the slide. · Dip
the slide in solution C for 30 seconds. Move the slide gently to and fro in
this solution. · Rinse
the slide clean with tap water by allowing the water to flow gently over the
back of the slide. · Apply
solution D for a contact period of 120 seconds; subsequently, rinse the slide
again with tap water. · Allow
the slide to dry and view with a 100x bright field objective. A blue filter
strengthens the contrast. Drying can be speeded up by first removing most of
the water with filter paper. Results
Gram negative Gram positive · The
solutions can be bought ready made. · Numerous
different recipes for Gram staining are mentioned in the literature. The recipe
described provides filamentous organisms with a good contrast. · Most
solutions can be retained for an almost unlimited time. Solution C (not the
stock solution) must be renewed once a month. · The
slides must be properly de-greased. · The
slides must be viewed with bright field. The difference between red and blue is
less clear with phase contrast. · The
slide must not contain too many sludge particles, as excess dye can then no
longer be removed by rinsing. Large 'blobs' of dye can be seen when viewing. If
this is the case, the staining must be repeated with fewer sludge particles on
the slide. |